Study Flexes Our Team’s Ingenuity
As part of an application for 510(k) approval, the U.S. Food and Drug Administration (FDA) requires assay developers to provide data on how their assay performs under a variety of less-than-optimal conditions. These tests are called flex studies and are generally standardized for all similar device submissions. For instance, will the assay still work after being exposed to high heat and humidity? Or after being dropped on the floor? Or if it is used and then sits exposed for two hours? Each of these conditions is relatively easy to recreate in a laboratory setting and then provides the customer with the necessary data to submit in their application.
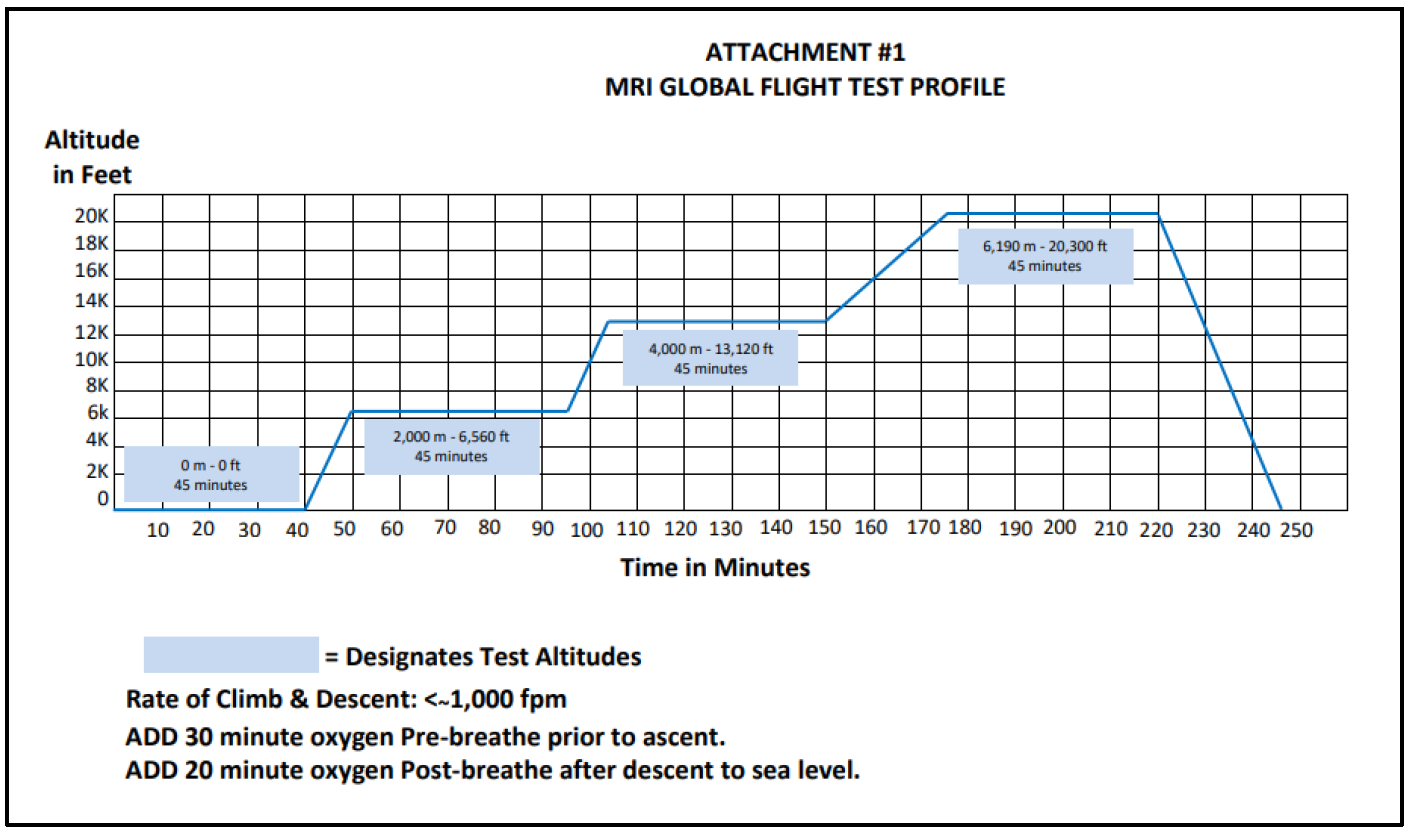
Our customer wanted to go further, interested in testing their assay in a way no company had ever even attempted. They challenged MRIGlobal to identify a solution under the more extreme conditions of an altitude flex study. This study required precision when detecting SARS-CoV-2 at various altitudes, confirming the assay’s accuracy to ensure detectability regardless of geographic location. Sitting only a few miles north of the Potomac River, our laboratories and team in Gaithersburg, Maryland, are well-positioned to perform a variety of work in the NCR region. But near sea level, they are most definitely not at the altitude required for this test. Instead of climbing a mountain with assays and laboratory equipment in their packs, our team sought out an innovative solution.

The team started by working in a glove box chamber with a vacuum system built in to pump air out of the unit, mimicking conditions at the desired altitudes. Unfortunately, the gloves in the box worn by the researchers would inflate due to the positive pressure inside the chamber, becoming bulky and rigid, making it impossible to effectively manipulate the assay and perform the entirety of the test “at altitude” inside the box. The team then attempted to utilize different types of gloves and create our own versions of gloves, but too much pressure built up behind the gloves and caused them to break the seal around the gasket and pop off. After assessing numerous other options that could not meet the demands of the test, they finally found one that did.
Our team of researchers contacted the manufacturer of training modules for pilots and astronauts to experience aspects of flight too dangerous to experience in flight, such as symptoms of hypoxia. They had a large, walk-in atmospheric pressure chamber that met the needs of this program, which we rented to perform the tests at simulated altitudes.
We began with a series of steps for members of our team that included biological safety preparations, a safety briefing, mask and helmet fitting, and laboratory preparations. Working with heat inactivated SARS-CoV-2, we then initiated the tests at simulated altitudes ranging from 4,000 to 20,000 feet.
What we found in conducting this research was that the assays performed the same at each of these altitudes, providing the customer with the data necessary to submit their 510(k) clearance application to the FDA.
GETTING STARTED AT MRIGLOBAL
Contact MRIGlobal to further understand our work in infectious diseases. We offer a broad portfolio of infectious disease tests and capabilities across diagnostic disciplines, from screening and diagnosis to genotyping, therapy, and monitoring. Those seeking analysis of infectious disease tests can trust in our breadth of experience and knowledge – not just on the subject matter, but on FDA protocols as well.
To learn more about the work we’ve done or how we can help you, contact us today. If you are part of an agency, business, or academic institution seeking assistance with a project, use our Project Quote Tool to get started.
SIGN UP FOR OUR NEWSLETTER
Sign up for the MRIGlobal newsletter! It’s the best way to get the latest updates in the world of applied scientific engineering research delivered directly to your inbox.

