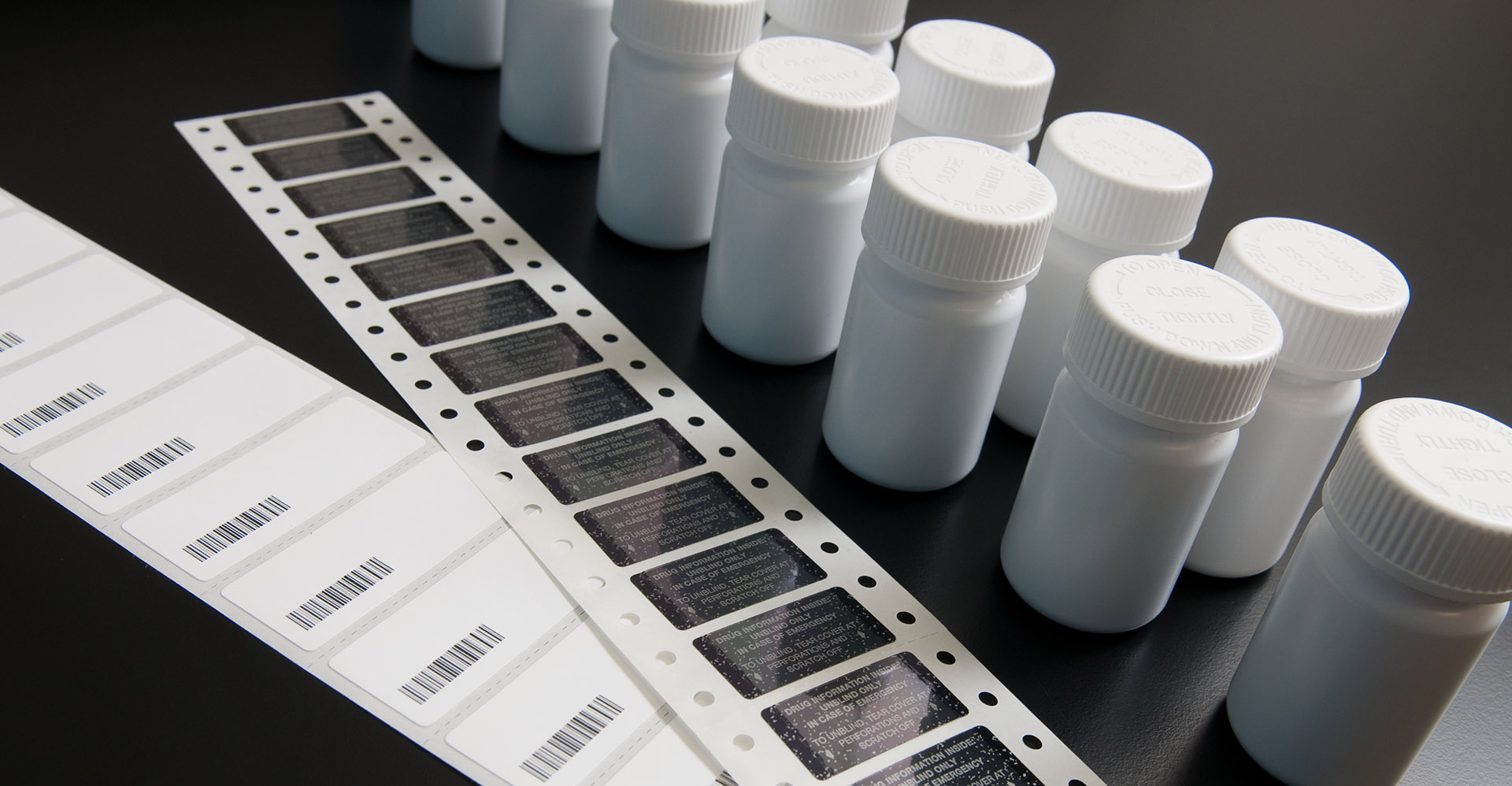
Biorepository
MRIGlobal’s biorepository collects, catalogs, and stores samples of biological material for research and pharmaceutical development.
MRIGlobal’s biorepository facility serves hold clinical and non-clinical biospecimens to meet our clients’ needs, as they study potential therapeutic drugs in research clinical trials. From toxicological testing to pharmaceutical development, MRIGlobal will ensure that properly stored and annotated material are available to you for your unique research endeavors. Our staff can also aid you in preparing your materials for storage, submitting analytical reports to regulatory agencies, and distributing your materials to researchers across the world. Accepted specimens include blood, plasma, urine, cells, DNA, proteins, and more.
Capabilities
MRIGlobal’s biorepository facility is your clinical solution for procurement, storage, and distribution of human or animal biological materials and tissue samples used for research, pharmaceutical development, clinical trials, and disease treatment.
MRIGlobal is your repository support solution for procurement, receipt, storage, and distribution of chemicals ranging from industrial compounds posing human health concerns to natural products, drug substances, drug products, and clinical trial materials.
Our auxiliary services include cGMP API and drug product manufacturing, analytical method development, validation and testing, and pharmaceutical shelf-life stability testing.
Our Staff
With decades of experience, our repository team includes a highly trained group of technicians, specialists and scientists who work diligently to accomplish project goals.
The group has extensive experience in all aspects of repository management including receipt, inventory management, packaging, labeling, and distribution. With a long-standing company-wide focus on safety, our team is trained in hazardous good shipments per DOT and IATA regulations to ensure all distribution activities are made in a proper manner. Our staff also understand the importance of regulatory compliance and espouse a culture of compliance, which is complemented by an experienced group of quality assurance officers. Overall, it is the MRIGlobal staff that make our projects successful, and we would be excited to see how our team can help your next project.

Work with us
MRIGlobal looks forward to contributing our expertise where it matters most.
Use our Project Quote Tool to see if MRIGlobal can help advance your next project.
