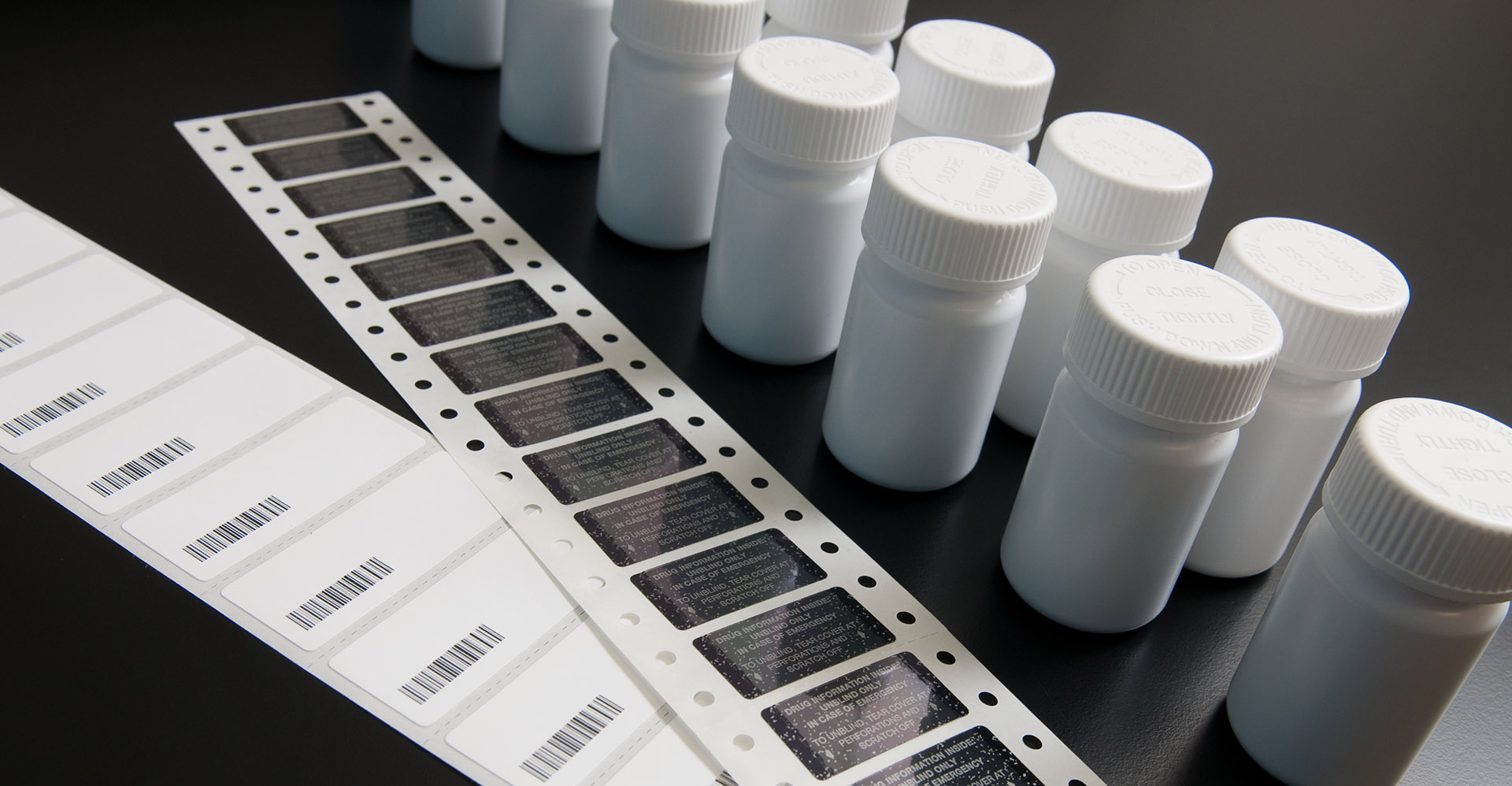
Exceeding Drug Product Quality Standards
Current Good Manufacturing Practices – cGMP’s – are the basis for meeting the drug product quality standards consumers expect.
With decades of experience, our unrivaled cGMP repository facility provides proper storage and distribution of drug substances and drug products to meet our client’s needs, as they study potential therapeutic drugs in research clinical trials. Our team can receive and store a wide range of investigational agents, including reference standards, small and large molecule biologic drug substances, bulk drug products, and clinical trial materials. With our manufacturing capabilities, we can also repackage and label investigational agents, and support blinded clinical trials through cGMP manufacturing of placebo and over-encapsulated comparator or investigational agents.
Additionally, this facility houses a Foreign-Trade Zone, enabling clients to send us product to store until it is approved by the FDA for use, while avoiding the uncertainty of international shipping.
Working with MRIGlobal gives you confidence in your product and your partner.
GETTING STARTED WITH MRIGLOBAL
Contact MRIGlobal to further understand our work in pharmaceutical sciences. We help clients develop faster bench-to-bedside processes to deliver therapeutic drugs to clinics for efficacy testing by bringing unique solutions to pharma from drug discovery to manufacturing.
To learn more about the work we’ve done or how we can help you, contact us today. If you are part of an agency, business, or academic institution seeking assistance with a project, use our Project Quote Tool to get started.
SIGN UP FOR OUR NEWSLETTER
Sign up for the MRIGlobal newsletter! It’s the best way to get the latest updates in the world of applied scientific engineering research delivered directly to your inbox.