End-to-End Clinical Trial Support for Material and Biospecimen Management by a Collaborative Team, in One Facility
MRIGlobal has proudly supported pharmaceutical development for decades, and in the area of clinical trial support, our North Kansas City facility provides unmatched convenience for our pharmaceutical clients. To further support their growing needs, we have recently expanded our repository, and added new services including a Foreign-Trade Zone and a biorepository. Each area is managed by a highly technically competent and experience team, and they are further supported by a well-established Quality Assurance program. These synergistic offerings broaden our ability to provide end-to-end support for clinical trials and ensure that we will meet customer needs for centralized services and convenience.
As investigational products are manufactured, MRIGlobal can support all receipt, storage, inventory management, and distribution needs as shown below. Whether the need is to hold, label, repackage and distribute clinical trial materials to clinical sites; or to receive, store and redistribute biospecimens from ongoing clinical investigations, MRIGlobal has it covered.
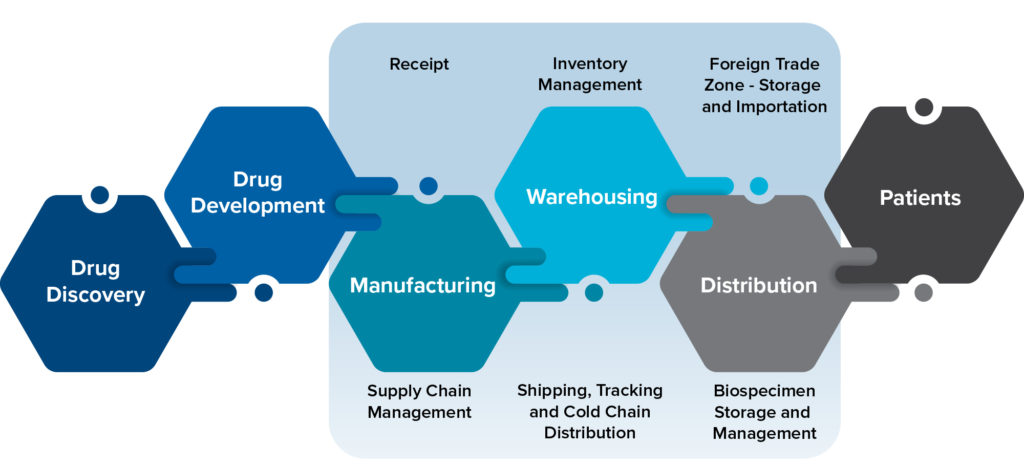
Repository
To support clinical trials, our recently expanded cGMP repository offers full-service management support and manufacturing services. With decades of experience, our team can receive and store a wide range of investigational agents, including small and large molecule (biologic) drug substances, bulk drug products, and clinical trial materials. With additional cGMP manufacturing capabilities, our group can repackage and label investigational agents, and support blinded clinical trials through cGMP manufacturing of placebo and over-encapsulated comparator or investigational agents. To complement our repository work, MRIGlobal also offers a full range of pharmaceutical analysis services. Our repository has recently been expanded to include additional storage capacity and we have also greatly expanded our pharmaceutical stability facility to meet the growing needs of our customers. Our repository is fully validated and operates in compliance with good storage and distribution practices and with cGMP regulations.
Moreover, given the importance of safety and security, our facility is equipped with inert fire suppression systems and fully redundant back-up power. Overall, the repository is fully capable of managing clinical trials needs from small early-stage trials to large later stage investigations with both domestic and international trial support.
This facility is capable of handling a range of materials such as cytotoxic and genotoxic materials, as well as light- and moisture-sensitive compounds. Additionally, the NKC facility has recently been established as a Foreign-Trade Zone (FTZ), allowing clients to send us product to store until it is approved by the FDA for use. This increased repository space will allow us to handle more projects and more customers, while establishing relationships with customers that have products in the FDA approval process.
Foreign-Trade Zone
In addition to the expansion of the repository, MRIGlobal has also recently licensed our site as a Foreign-Trade Zone (FTZ), operating in compliance with the Foreign Trade Zones Act 19 and 15 CFR Part 400. This facility can be the first stop when shipping materials that are not yet approved by the FDA into this country. This designation provides clients with numerous advantages, including avoiding the uncertainty of international shipping, and/or deferring duty payments and even eliminating duties in some instances.
This unique offering is appealing to customers across the industry, but especially those small or virtual pharmaceutical companies who may manufacture overseas and have a need to move the materials to the United States, but do not have an approved IND nor have access to space of their own. The FTZ is in the same facility as our newly expanded repository and recently established biorepository, so materials are easily imported once approved. Upon arrival, materials are received by MRIGlobal at the designated Port of Entry, admitted to the FTZ, and transported by bonded truck to the Repository where the material will be stored in the specified storage conditions within the designated FTZ spaces. The material will be held in the FTZ until directed by the client to either import the material or ship it to another country.
Importing within the MRIGlobal repository involves completing the import process with our designated FTZ broker. Once imported, moving the material from the FTZ spaces within the repository to the U.S. spaces of the repository. The material can be held in U.S. spaces within the repository for distribution or shipped to another U.S. company after completion of the import process. If the material is being shipped outside of the U.S., the material will be transported by bonded truck to the port of entry where it will be released from the FTZ and shipped to the specified foreign country.
Biorepository
From researchers performing clinical trials, to those conducting cutting edge discovery research, storage and annotation of biospecimens is a critical need. For customers in need of a full-service biorepository that does more than simply store samples, MRIGlobal’s biorepository can help.
The purpose of the biorepository is to maintain the specimens – such as blood, urine, tissues, cells, DNA, RNA, and protein – under controlled conditions in support of current and ongoing studies or for future reference and research purposes. From an operational standpoint, we can receive samples from points of collection, which are often clinical trials. We then process the samples, store and inventory them, and ultimately distribute them back to researchers.
Because these samples are very valuable, and in many cases irreplaceable, MRIGlobal prioritizes our control and management systems. Through our robust standard operating procedures, utilization of highly trained staff, and adherence to biorepository best practices such as ISBER and NCI Biorepository best practices, MRIGlobal works diligently to ensure that all samples are maintained appropriately.
While other biorepositories are available only for sample storage, we take pride in offering a full suite of services, with the capabilities to perform end-to-end services – not just in the biospecimen space, but for all research and clinical trials needed, including in-house analysis and annotation of samples. Whether our clients need cGMP API, drug product manufacturing, analytical chemistry, clinical trial material management and/or biospecimen support, MRIGlobal can provide it all. We aim to partner with our clients, ensuring success. Together, we will solve the world’s most important challenges and our biorepository is an important element of the solution.

GETTING STARTED WITH MRIGLOBAL
As a not-for-profit contract research organization, we have proven ourselves as an objective partner driven to make our clients’ products more successful. We specialize in defense, human health, pharmaceutical sciences, in-vitro diagnostics, energy and environment, agriculture, and global health.
To learn more about the work we’ve done or how we can help you, contact us today. If you are part of an agency, business, or academic institution seeking assistance with a project, use our Project Quote Tool to get started.
SIGN UP FOR OUR NEWSLETTER
Sign up for the MRIGlobal newsletter! It’s the best way to get the latest updates in the world of applied scientific and engineering research delivered directly to your inbox.

