Biocontainment is crucial to biorisk management and avoiding further exposure of infectious microorganisms to the greater population. However, while many medical centers may have a biocontainment unit within the facility, up to now there hasn’t been a system that could sustain air travel without compromising its ability to care for patients.
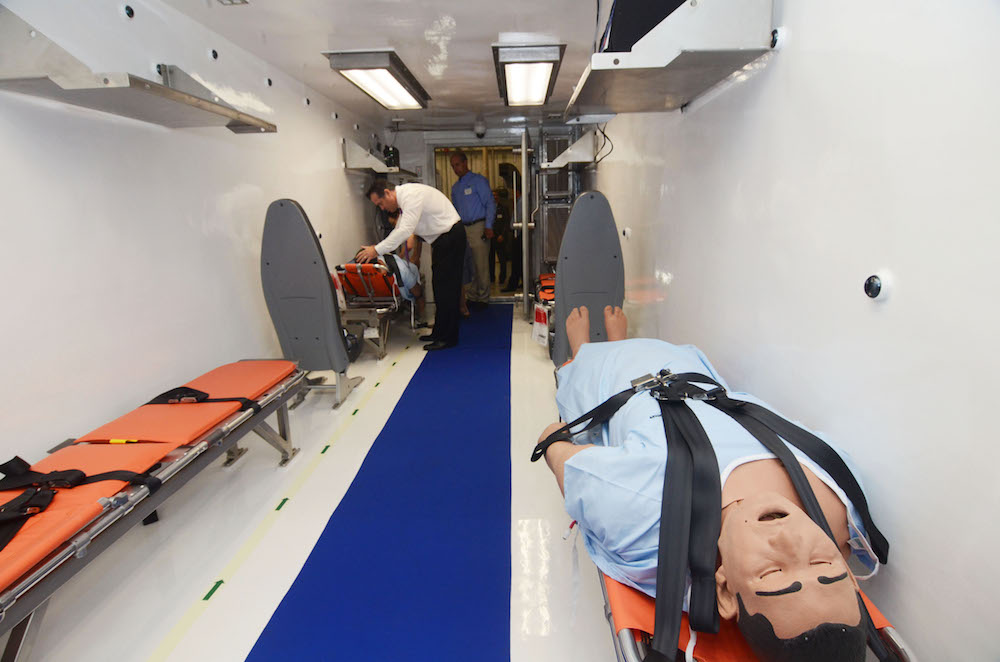
As an international leader in infectious disease, MRIGlobal created the Containerized Bio-Containment System (CBCS), a flyable medical transport unit—the first of its kind. Designed for high containment, this system features three rooms and fits four patients and four caregivers within a single system.
MRIGlobal is an independent contract research organization specializing in defense, human health, pharmaceutical sciences, in-vitro diagnostics, energy and transportation, agriculture, and global health. Our solution-driven teams are dedicated to finding solutions for a safer, healthier, more sustainable world.
Learn more about our innovation system and how the CBCS changes the future of biocontainment technology across the globe.
Mobile Biocontainment Quick Facts →
How the Containerized Bio-Containment System emerged
The CBCS emerged to fulfill a need of the United States Department of State with funds provided by The Paul G. Allen Foundation. This was during the Ebola outbreak in West Africa and the Government needed a way to evacuate people from infectious disease areas to the U.S. From this need, the Department of State gave us three main requirements:
- The system needed to hold at least four patients and four caregivers in a biocontainment environment
- The system needed to facilitate medical care during air travel
- The system would operate on a commercial 747 or military C-17 cargo aircraft
The main issue: No one knew if this was possible. Finding solutions for the seemingly impossible is what MRIGlobal is known for. After the initial request, we spent two weeks devising the design for the Department of State and The Paul G. Allen Foundation.
Within that short period, they received our design then performed a global search for other contractors who could possibly build the same thing. From months of searching, they found that MRIGlobal’s design was the best option. The choice came down to the execution of the design, which is where our team’s talent came into play.
Since the Ebola outbreak was a major concern and a global threat, we didn’t have much time to fabricate the CBCS. In fact, we only had 191 days. Our engineers and other team members spent days, nights, and even holidays to make sure we could get the systems completed in time. It was truly a 24/7 effort for a lot of people to make it all happen, a feat that was necessary during the 2014 epidemic.
CBCS current and potential future applications
While we initially designed the CBCS for the Ebola outbreak, its application is much greater than the Ebola virus. We anticipate that government and private institutions could deploy the CBCS in a variety of situations. Here are two examples.
The first use would be similar to how it was used in West Africa. The unit could load onto an aircraft for biocontainment from the patients inside with highly infectious disease. Ideally this use would not be reserved solely for the United States government. Foreign governments could benefit from their own Containerized Bio-Containment Systems.
Because epidemics can easily grow to a global scale (as we’ve seen with the latest COVID-19 outbreak), biocontainment is a world-wide effort. Having commonality of systems that easily transport via aircraft, moving patients to adequate medical care will be a concern to all governments, not just the United States.
The second use would be more as a ground-based unit for hospitals. It would act as an extension of a triage unit outside of the actual building. In this scenario, you could quarantine patients when necessary rather than have them enter a hospital where two or three patients could potentially take up an entire wing. We believe that there are times where it could be more appropriate to implement a CBCS and have a patient stay inside for a particular amount of time in order to keep the hospital open.
Get to know the CBCS: Frequently Asked Questions
We recently sat down with Dr. Dean Gray, Director of MRIGlobal’s National Security and Defense Program. He was involved in the project from inception, through design, fabrication, and deployment of the Containerized Bio-Containment System. Here are some common questions he receives about the unit.
How did MRIGlobal test the equipment when creating the CBCS?
We put our equipment through a lot of computer-simulated modeling up-front to determine whether or not our design could work. We also physically tested and evaluated the system at different stages of the construction process. Because our first systems were designed for the U.S. government, there were very strict requirements on the certification and other aspects of the unit—even down to the type of bolts we could use for fabrication. That means testing had to be very meticulous and tightly controlled.
After I send a RFP, how soon can I expect my CBCS?
From beginning to end it takes four months to build a CBCS, and we generally build them in pairs. The idea is that you would request your CBCS before an emergency arises. For instance, ideally several CDC hospitals would have multiple CBCS units close by and ready to deploy when needed. When you plan ahead, you won’t have to worry about waiting months for fabrication when the need is immediate.
Is MRIGlobal available for operations assistance after delivery?
After delivery, the Department of State had another contractor to handle the emergency air operations outside of Atlanta in coordination with the CDC. This will be the general set up for future customers as well. Once we have gone through design, fabrication, check over, and delivery, we have no further input in CBCS operations.
What kind of maintenance can I expect?
The CBCS comes with a battery backup system, so if the airplane were to lose power or the CBCS had to sit on a tarmac for a certain amount of time, the battery power would keep all the systems up and going for about four hours. Maintaining proper battery life is one focus of routine maintenance.
Outside of battery maintenance, the HEPA filters should be routinely inspected and replaced if necessary.
What is the expected turnaround time for reuse?
When thinking about the turnaround process, you have to consider the ability to properly decontaminate the system. This is accomplished by introducing vaporized hydrogen peroxide (VHP) throughout the system. In most cases it will take a few hours to decontaminate every surface.
A unique feature of the CBCS is that the interior is coated with a continuous surface of impermeable epoxy, meaning there are no corners. When VHP hits the surface, there isn’t any place for a virus or other microorganism to hide in that system. This makes complete decontamination possible.
Benefits of working with MRIGlobal
There really isn’t a lot we can’t do or won’t attempt to do, which is why many of our customers come to us in search of innovative solutions. Everyone involved all the way up to our CEO is excited about solving the problems that have no clear answers.
Regardless of the industry or department, customers come to us asking, “Is this possible?” Oftentimes through our experience and research, we find that the answer is typically yes and we discover ways to go about it. From there we create a schedule, budget, and clear timeline to assure our customers that we will do what we say we can do.
Our drive, along with disciplined program management, is how we deliver the highest quality products and build a credible reputation around the world.
Learn more about our Containerized Bio-Containment Systems
For more than 75 years, MRIGlobal has advanced scientific research across a variety of industries. We focus on supporting better health outcomes on a global scale. For us, it’s not simply about science; it’s about the people who make it all happen.
We have a solution-driven mentality that drives us to solutions for even the most complex situations. Through our dedication, we can solve any technical problem.
To learn more about the Containerized Bio-Containment System, contact us at info@mriglobal.org. You can also get a free quote by completing our online form.
If you’re part of an agency, business, or academic institution seeking help with a project, use our Project Quote Tool to get started today.

